 The short answer is no. Despite what you may have heard some snowflakes are exactly the same shape and size as other snowflakes, at least to the naked eye.
The short answer is no. Despite what you may have heard some snowflakes are exactly the same shape and size as other snowflakes, at least to the naked eye.
The long answer follows: Jon Nelson, a researcher with Ritsumeikan University in Japan, has studied snowflakes for fifteen years, and has some interesting insights into their delicate structures. He points out that the old adage that ‘no two snowflakes are alike’ might be true for larger snowflakes, but it does not hold true for smaller, simpler crystals that fall before they’ve had a chance to fully develop into the familiarly evocative hexagonal flakes. Regardless, the shape of snow crystals are incredibly diverse, this is partly due to their sensitivity to even the smallest temperature change as they fall through the clouds.
So, how do snowflakes form in the first place?
Put simply, at the heart of every snowflake is a minute grain of dust that was once floating in a cloud. Water vapor from the atmosphere condenses on this dust grain forming a droplet that freezes instantly (it’s a nucleation process to put it technically).
The ice crystals grow with hexagonal symmetry. The shape originates from the chemistry of the water molecule, which consists of two hydrogen atoms bonded to an oxygen atom, H-O-H. These atoms are not in a straight line though, they’re at an angle and this angle means that when several water molecules get hitched together through hydrogen-bonding in the frozen state the simplest way to do that (i.e. the lowest-energy arrangement) results in six-sided symmetry, hence six-sided snowflakes. By the way, you never get octagonal or pentagonal snowflakes, please report anyone who draws or makes snowy decorations with flakes showing that symmetry.
The growing flake eventually sprouts six tiny branches. Each of these branches grows to form side branches in a direction and shape that are influenced by the clustering of water molecules on the ice crystal surfaces.
The American Chemical Society has produced an excellent poster illustrating all this. You can download it as a PDF file here.
You may be wondering why scientists are so interested in snowflakes, after all in many parts of the world, you’re never going to get a chance to test your theories.
Well, snowflakes and ice crystals have an effect on the global climate because of the reflectivity, they are thought to help catalyze the break down of ozone in the upper atmosphere depletion, and they also play a critical role in the build up electric charges in clouds that leads to lightning.
The Scout Report recently highlighted another researcher with an interest in ice crystal growth and pattern formation in ice. Kenneth Libbrecht at Caltech University, the Report says, is “so interested in fact, he went
ahead and created this lovely website that documents the very wide, and very
interesting world, of ‘snowflakes, snow crystals, and other ice phenomena.’
But, that’s probably enough science for today. So, wherever you are let it snow, let it snow, let it snow…and compliments of the season from Sciencebase.com
 Over at the American Institute of Physics my colleague Phillip Schewe and his team have been putting together their pick of the physics discoveries for 2006.
Over at the American Institute of Physics my colleague Phillip Schewe and his team have been putting together their pick of the physics discoveries for 2006.


 It’s Boxing Day and you’re probably seriously bored playing the “normal” game of Pick-up Monkeys. Rather than heading for the Wii or the PS3, how about adding a little monkey magic, or more seriously some wire binders and following Dr N. Michael Green, Division of Mathematical Biology, of the UK’s prestigious Medical Research Council (MRC) National Institute for Medical Research to do a little bit of science education with those colourful plastic monkeys.
It’s Boxing Day and you’re probably seriously bored playing the “normal” game of Pick-up Monkeys. Rather than heading for the Wii or the PS3, how about adding a little monkey magic, or more seriously some wire binders and following Dr N. Michael Green, Division of Mathematical Biology, of the UK’s prestigious Medical Research Council (MRC) National Institute for Medical Research to do a little bit of science education with those colourful plastic monkeys. What on earth’s a “booyle rocket”, I hear you ask! Well, I haven’t a clue. It’s just a search term that a Sciencebase visitor used in our search box.
What on earth’s a “booyle rocket”, I hear you ask! Well, I haven’t a clue. It’s just a search term that a Sciencebase visitor used in our search box. The short answer is no. Despite what you may have heard some snowflakes are exactly the same shape and size as other snowflakes, at least to the naked eye.
The short answer is no. Despite what you may have heard some snowflakes are exactly the same shape and size as other snowflakes, at least to the naked eye.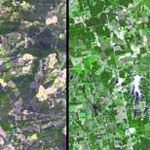 A couple of years ago NASA released satellite images of Santa Claus’ summer hideaways, turns out there are two locations in the USA where old Father Christmas likes to spend the lazy summer months. Santa Claus, Georgia (left) and Santa Claus, Indiana (right), are typical of Santa’s summer retreats, says NASA, offering Santa and Mrs Claus, all the reindeer and elves the usual amenities and a nice warm respite from their chilly Polar home during the off season.
A couple of years ago NASA released satellite images of Santa Claus’ summer hideaways, turns out there are two locations in the USA where old Father Christmas likes to spend the lazy summer months. Santa Claus, Georgia (left) and Santa Claus, Indiana (right), are typical of Santa’s summer retreats, says NASA, offering Santa and Mrs Claus, all the reindeer and elves the usual amenities and a nice warm respite from their chilly Polar home during the off season. Anyway, the official address to send your Letter to Santa is: Mr S Claus, North Pole, H0H OH 0, don’t worry if that zipcode looks a little odd, just try saying it out loud and you’ll feel okay.
Anyway, the official address to send your Letter to Santa is: Mr S Claus, North Pole, H0H OH 0, don’t worry if that zipcode looks a little odd, just try saying it out loud and you’ll feel okay. Many people in the modern Western world delude themselves that their culture is generally free from the effects of intoxicating substances except in the criminal underworld, and that ‘nice people don’t take drugs’. But Richard Rudgley of the University of Oxford, a researcher of the oasis communities of Chinese Central Asia, shows that our culture and other cultures across the world have a rich tradition of using chemicals, mainly from plants, to produce altered states of consciousness. These range from the ritualistic use of the fly-agaric in Palaeolithic Europe to betel-nut chewing in Papua New Guinea, and from pretentious bone-china tea sets in Surbiton to the tragic inhaling of petrol fumes by Aboriginal Australians.
Many people in the modern Western world delude themselves that their culture is generally free from the effects of intoxicating substances except in the criminal underworld, and that ‘nice people don’t take drugs’. But Richard Rudgley of the University of Oxford, a researcher of the oasis communities of Chinese Central Asia, shows that our culture and other cultures across the world have a rich tradition of using chemicals, mainly from plants, to produce altered states of consciousness. These range from the ritualistic use of the fly-agaric in Palaeolithic Europe to betel-nut chewing in Papua New Guinea, and from pretentious bone-china tea sets in Surbiton to the tragic inhaling of petrol fumes by Aboriginal Australians. An advanced knowledge of electromagnetic waves, the space-time continuum, nanotechnology, genetic engineering, and computer science easily explains Santa’s abilities to deliver presents to millions of homes and children in just one night, according to professor of mechanical and aerospace engineering, Larry Silverberg, at North Carolina State University.
An advanced knowledge of electromagnetic waves, the space-time continuum, nanotechnology, genetic engineering, and computer science easily explains Santa’s abilities to deliver presents to millions of homes and children in just one night, according to professor of mechanical and aerospace engineering, Larry Silverberg, at North Carolina State University.