You’ve seen the kind of thing: “Warehouse Razed to the Ground in Fire”, as if razing didn’t already mean the building was levelled. Worse, “Balloon Ascends Up into the Air”, ascending down is very difficult, simultaneously, at the same time, if not impossible; so too is descending up.
However, the award for the most redundantly tautological headline of the year has to go to Scientific American for Male Semen Makes HIV More Potent, that’s male semen as opposed to the female variety, is it? It’s an important discovery, nevertheless that a chemical constituent of semen affects the immune system facilitating viral infection.
Scientific American is probably not the first and original nor the ultimate and last publication to use this phrase though. DoctorNDTV ran a story with the title: Male semen loss concerns and risky sexual behaviour. Then there’s a research paper in the Journal of Avian Biology that discusses bacteria found in the “male semen” of red-winged blackbirds. Even the venerable and well-respected New Scientist recently published an item on insect courtship and egg laying. Apparently, the trigger for egg laying “is a small protein called sex peptide (SP) in the male’s semen.” Again, the word male, while perhaps making the sentence smoother, is totally redundant and not needed.
A search for the phrase “male semen” on PubMed produced not hits, although “male sperm” came up several times in various journals. So as not to appear sexist, I also did the equivalent searches for “female semen” and “female sperm” and quite surprisingly got several PubMed hits. One paper on mythology mentions how at one time in human history a godly being or other supernatural entity was thought to intervene in the merging of male and female semen to bring about conception. Not quite a modern biomedical reference point, then. The phrase “female sperm” gave absolutely no hits, unsurprisingly.
Maybe the clue as to why these various publications qualify the word semen lies in those papers discussing the mythology of reproduction. A quick Google shows that there are many references to religious and proto-religious texts that discuss both male and female semen as if they were both real. Perhaps by qualifying semen as male in modern writing, rather than simply discussing semen, there is some referential nod to humanity’s misconstrued understanding of reproduction. But, modern understanding of reproductive biology defines semen as a product of the male reproductive organs that acts as a transport medium for sperm, so, like I said, it’s redundant.
I asked linguistic guru Steven Pinker of Harvard University, whose book The Stuff of Thought I reviewed on Sciencebase recently, about this apparent paradox. Pinker told me that he suspects that, “the cause is not a nod to the ancients, but a desire to call the reader’s attention to the fact that it’s
the naturally occurring fluid that encourages the potency of the virus, not some externally administered product.
“Semen Makes HIV More Potent implies to me,” he said, “that adding semen increases the potency, rather than that the HIV exploits the properties of the semen it finds itself in.” He adds that it is peculiar that this may be the case. “Odd that the redundancy should do that,” he told me, “but somehow I think it does.”
Intriguingly, after I contacted Pinker, I saw that the journal Nature, as opposed to the popular science magazine, Scientific American, had covered the same story. In Nature, however, their piece was entitled – Semen boosts HIV transmission. So, for some reason they felt semen does not need a masculine qualification of any kind. The tautology of the phrase “male semen” may seem trivial, but it is an important issue.

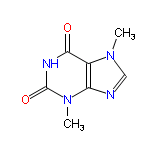
 So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.
So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.