 A new(ish) website has launched that aims to provide unbiased patient-generated data on the benefits of 7000 prescription medications and their side-effects.
A new(ish) website has launched that aims to provide unbiased patient-generated data on the benefits of 7000 prescription medications and their side-effects.
Rateadrug.com hopes to do for pharma products what dooyoo and ciao do for gadgets by bringing the crowd to the debate. Patients can anonymously rate and review any of the prescription drugs they take and view other people’s experiences for free.
“All information on this site is unique, community data that is not biased by pharmaceutical or corporate objectives,” says spokesman Jack Dowd. He adds that, “The site provides patients with truly independent survey results about the risks and benefits of their medications. The more people that start using the site to rate their prescription medications (a quick 5-minute survey), the greater this resource will become.”
Using a prescription drug appropriate to your condition and your genetics can have significant, and often life-saving benefits, but with physicians particularly in the UK and a few other places emphasising how patients should help manage their own illness it is important to know what problems may arise or whether asking for a different prescription might actually be better for them.
Not all medications hit everyone in the same way, because of various factors including your SNPs (single nucleotide polymorphisms), that affect your body’s enzyme and receptor activity. “What’s effective and side-effect free for one person might not be the best drug for someone else, yet most harried doctors prescribe the same drug for 90% of their patients with similar conditions – regardless of individual sensitivities,” the site’s developers say. They hope that Rateadrug will prevent the next Vioxx from happening.
A side project of Rateadrug is involving pre-med students through the PreMed Prescription Rating and Experience Program (PREPP) where the students help senior citizens become more proactive with their drug intake by reporting their experiences through Rateadrug.com.
The site blurb suggests you use the reviews together with your doctor’s advice and FDA disclosures to achieve the best possible outcome for your (or your loved ones) medical condition. Of course, spammers and corporate shills will be readying themselves to distort the results in their favour, unless preventative measures are put in place. “To prevent spam and ensure a real person is taking each survey we require email verification where the user has to click on a link that we email to them,” Dowd told Sciencebase, “We also flag accounts that submit more than one rating for a specific drug. We’re committed to providing quality, real-user data and will continue to ensure that our results are not skewed by spam or anyone trying to influence the results of a specific drug.”
The site also drops a cookie on to your machine so that it knows how many people log in from a specific computer or IP address. If it looks like there are a lot of ratings for the same drug from the same IP address, they will flag those ratings for manual checking.
I asked Dowd to expand on how they are addressing security and validity issues. “At the moment we receive 20 or fewer ratings per day, and carefully review each one,” adds Dowd, “As the volume increases significantly it will become more difficult to impact and distort results – real ratings should outweigh any attempts to skew ratings. But, we will do our best to prevent this type of tampering.”
“Right now, we have a database of over 7000 drugs, but only about 300 have been rated and reviewed by users/patients,” he adds. Dowd and his colleagues hope that as more people find out about this site, the numbers will grow. “Our intention is to provide real ratings by real people and will do everything we can to assure this as we progress,” he told me.
According to CEO Mark Deuitch, RateADrug is currently hoping to get large numbers of patients to review the cholesterol-lowering statin drugs Lipitor, Lescol, Mevacor, Pravachol, and Zocar, anti-depressants such as Lexapro, Prozac, Effexor, Paxil, Zoloft, and Pristiq, and drugs used to treat insomnia including Ambien, Lunesta, Sonata, Rozerem, and Benzodiazepines.
In related news from the UK’s National Health Service: Drug reference information in the British National Formulary will become a key element of the new NHS Evidence portal due to be launched in April 2009. As a result, responsibility for provision of this information for the NHS will transfer from the Department of Health to the National Institute for Health and Clinical Excellence (NICE), as part of the development of NHS Evidence.
 This week, The Alchemist is digging in the dirt to find out about the carbon cycle and climate change, taking his whisky (or is it whiskey) with or without water, and discovering how to juggle molecules, on the other hand. Also in biochemical news this week, the crystal structure of a plant hormone receptor is revealed while researchers in Israel focus on blocking the protein misfolding that occurs in Alzheimer’s disease.
This week, The Alchemist is digging in the dirt to find out about the carbon cycle and climate change, taking his whisky (or is it whiskey) with or without water, and discovering how to juggle molecules, on the other hand. Also in biochemical news this week, the crystal structure of a plant hormone receptor is revealed while researchers in Israel focus on blocking the protein misfolding that occurs in Alzheimer’s disease. A new(ish) website has launched that aims to provide unbiased patient-generated data on the benefits of 7000 prescription medications and their side-effects.
A new(ish) website has launched that aims to provide unbiased patient-generated data on the benefits of 7000 prescription medications and their side-effects. A while back, I visited the Bushmills whiskey distillery (it was my second visit in as many decades) always a pleasure, especially the tasting panel at the end of the tour just before you spend all your money on, ahem, souvenirs.
A while back, I visited the Bushmills whiskey distillery (it was my second visit in as many decades) always a pleasure, especially the tasting panel at the end of the tour just before you spend all your money on, ahem, souvenirs.
 My latest science news is now online in the spectroscopyNOW ezine. This week:
My latest science news is now online in the spectroscopyNOW ezine. This week: Congratulations to
Congratulations to 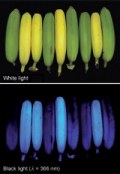 Also, this week “Yes, we have blue bananas!” – Forget the so-called
Also, this week “Yes, we have blue bananas!” – Forget the so-called  In the latest
In the latest  In environmental news, recent insights into dust from the Sahara could improve our understanding of climate change. Finally, dust of another kind is being used in an entirely different way, by British researchers to protect a new type of thermometer used to measure the 3000 Kelvin temperatures of an explosion.
In environmental news, recent insights into dust from the Sahara could improve our understanding of climate change. Finally, dust of another kind is being used in an entirely different way, by British researchers to protect a new type of thermometer used to measure the 3000 Kelvin temperatures of an explosion.