‘Twas the night before Christmas, when all through the house
Not a creature was stirring, not even a mouse
Well, that may not be quite true, do you know what your dog is doing right now? What about the cat? They’re not rummaging through the presents under the tree are they? You didn’t leave any luxury plain or dark chocolates under there did you? If you did, you could wake up to a quite horrible surprise when Christmas morning comes around. Chocolate makes pets ill! That’s the important seasonal warning being delivered by vets everywhere.
 So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.
So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.
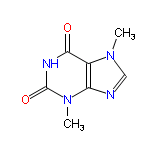
Theobromine has nothing to do with the element bromine, rather it is a bitter alkaloid from the seed of the cacao tree, known scientifically as Theobroma. The basic chemical skeleton is a xanthine unit, same one on which several other more well-known natural chemicals are based. The stimulant caffeine (from coffee beans) is a xanthine, for instance, so too is theophylline (found in tea and used in treating COPD and asthma).

Lacking the enzymes to metabolize theobromine leaves dogs exposed to the toxic action of the compound, which can cause vomiting, diarrhoea, hyperactivity, tremors, seizures, abnormal heart rhythms, and in extreme cases death.
So, what are the first signs of chocolate poisoning, what’s the lethal toxic dose, and what should you do if you think your dog has been at the chocolatier’s produce? Well, the first signs of chocolate poisoning are vomiting and diarrhoea, increased frequency of urination, and nausea. A toxic dose that will cause symptoms is about 100-150 milligrams of theobromine per kilogram of body weight, toxic dose could be approximately 250 and 500 mg/kg. A kilogram of milk chocolate will contain about 2 grams of theobromine. So a greedy, little dog is at greater risk of a serious digestive upset than a big gluttonous dog if he or she snaffles a 250 g bar of milk chocolate.
There is no direct way to treat chocolate poisoning in dogs, although inducing vomiting if the animal is caught “brown-pawed” (best left to the vet) is probably a good idea. A dog that has eaten a lot of chocolate will almost certainly require hospitalisation for at least the four days while the theobromine is naturally excreted (It takes about 17 hours for a dog to excrete half of the theobromine in its system, which means this toxin will reach vital organs repeatedly via the blood stream). But, who needs that, or worse, at Christmas? As they say: a gram of prevention is worth a kilo of cure.
For lovers of cats (small, domestic, big, wild) keep the aspirin out of reach. It’s deadly poisonous to felines.



 So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.
So, what’s the problem? Surely a little chocolate snack in the night isn’t going to harm good-old Fido? Well, our vet begs to differ and so do the veterinary toxicology sheets for the chocoholics’ favourite fast-food. Man’s best friend, and several other animals, you see lack a particular liver enzyme that their owners do possess, that breaks down a toxin found naturally in chocolate – theobromine.