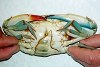
David Johnson and Robert Watson thought they had seen all there was to see in the Chesapeake Bay in almost three decades until they pulled out a crab from the way that had a male left half and a female right half. Now, that crab, acquired by Romuald Lipcius of the Virginia Institute of Marine Science at the College of William & Mary, has moved sideways into the world of natural metabolites where its gynandromorphic peculiarities have helped scientists, for the first time, discover that some molecules can be made only by one sex and not the other.
The male-female crab is a unique example of the blue crabs. It turns out that the males of this species produce a natural metabolite that is absent in females. This suggests that some complex biochemistry is underway that is activated only in males. Robert Kleps of the University of Illinois at Chicago and colleagues have isolated this small molecule and identified it as 2-aminoethylphosphonic acid (AEP), an uncommon but well-documented natural metabolite.
We used low-field NMR using phosphorus-31, to observe the small molecule, explains Kleps. He points out that science tends to get lost in the rush for higher field NMR running hydrogen-1 and carbon-13 on 100 kilodalton proteins. However, he adds that, “Even low-field NMR spectroscopists can make interesting discoveries. I’m very happy to have stumbled over this metabolite, while doing basic research on invertebrate metabolism.”
So, why might the existence of a metabolite in the males of this blue crab species and not the females have any bearing on our everyday lives? Well, there are well known differences between the sexes in people, such as disease susceptibility, anatomy and drug metabolism. Kleps points out that these differences might in fact be due to the presence or absence of a crucial metabolite.
Now that the existence of a sex-specific metabolite has been found for one animal the search is on for others, including ones that might exist in people.
For more details on the NMR study check out my column on SpectroscopyNOW.com, the research paper itself is available in Plos One.
You can hear a description of the crab from Lipcius here and listen to Kleps’ podcast