 Sciencebase reader Toar Winter emailed with a rather intriguing question.
Sciencebase reader Toar Winter emailed with a rather intriguing question.
Baseball star Rafael Palmeiro tested positive for anabolic steroids in August 2005 after telling the US Congress he had never taken such drugs before and was banned for ten days. He still insists he did not take steroids, or that if he did they were ingested through unprescribed supplements that contained stanozolol, the actual substance for which he tested positive.
Stanozolol is an old steroid and a test for its illegal use in sport has been used successfully for many years. It would actually make little sense for any sportsperson to cheat using this particular drug as it is detected so easily. This is especially true given the plethora of alternative, including human growth hormon (HGH) for which current testing methods are not quite so efficient.
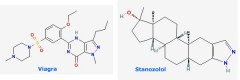
So, back to Winter’s question. First, he points out that Palmeiro’s only advertising sponsor is Viagra, then asks whether or not it could be Viagra that produced a “false positive” for the steroid. After all, there is certainly some overlap between the effects and side effects of viagra and certain steroids. Winter, wants to know whether or not Pfizer in developing Viagra initially altered the structure of stanozolol slightly to produce a ‘different’ drug with similar or more directed effects.
As far as I know this is not the case. Pfizer was developing a drug for angina and high blood pressure in the late 1980s when it discovered that male volunteers in its clinical trials were seeing a rather outstanding side-effect of the drug. They’d actually based their leads on a known, but unmarketed, allergy drug Zaprinast, which inhibits the enzyme phosphodiesterase. The rest is history. This compound is not related to stanozolol as far as I know. If any readers can affirm otherwise, please leave a comment or email me.
Winter’s line of reasoning, however, is intriguing nonetheless. “Think about it,” he says, “not many athletes in today’s world are of the age where Viagra is needed, so drug testing probably wouldn’t detect many offenders.
It’s an intriguing idea, but I don’t think Winter’s theory stands up to close scrutiny. If it did, wouldn’t it be ironic that many of those same (elder) Congressmen pointing the finger at Palmeiro would have also shown positive in the same test?
Meanwhile, in other news, Pfizer has lost its battle to name the Chinese brand Viagra, “Wei Ge” (according to http://www.chinacourt.org/). The phrase is commonly used in China, where copycat generics out sell the real thing, but Guangzhou Welman, a Chinese ED drug maker already registered this name and the courts have said it should be allowed to retain the rights to it. I’m sure at least one regular reader of the Sciencebase blog will be able to tell us the origin of that name…
 Bottled seaside air! It almost sounds like a scam from the Victorian era when the bracing “ozone” of fresh air at the British seaside was said to cure all kinds of ailments and led to a boom in seaside resorts and continues to ebb and flow.
Bottled seaside air! It almost sounds like a scam from the Victorian era when the bracing “ozone” of fresh air at the British seaside was said to cure all kinds of ailments and led to a boom in seaside resorts and continues to ebb and flow.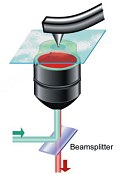 Swiss chemists have devised a new approach to the familiar analytical technique of Raman spectroscopy that allows them to investigate the structure of individual molecules.
Swiss chemists have devised a new approach to the familiar analytical technique of Raman spectroscopy that allows them to investigate the structure of individual molecules. Advocates of nuclear power point to recent advances in waste storage materials that could allow the radioactive byproducts of the nuclear industry to be stored safely and indefinitely in ceramics rather than glass. Whereas those not in favour of splitting atoms to produce almost limitless energy point out that even vitrified nuclear waste will represent an ongoing problem for thousands of years.
Advocates of nuclear power point to recent advances in waste storage materials that could allow the radioactive byproducts of the nuclear industry to be stored safely and indefinitely in ceramics rather than glass. Whereas those not in favour of splitting atoms to produce almost limitless energy point out that even vitrified nuclear waste will represent an ongoing problem for thousands of years. Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.
Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.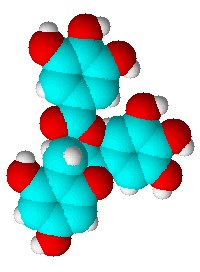 Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria.
Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria. A simple Q tip is all it takes to grab a microscopic sample from a work of art for laboratory testing, according to Canadian analytical chemists. They’ve used the approach to sample darkening pigments from an ancient map and from a piece of modern art as proof of principle.
A simple Q tip is all it takes to grab a microscopic sample from a work of art for laboratory testing, according to Canadian analytical chemists. They’ve used the approach to sample darkening pigments from an ancient map and from a piece of modern art as proof of principle.