Raman spectroscopy, X-ray diffraction, and electron microscopy could help diesel engine components manufacturers meet tough new emissions regulations, according to researchers at Oak Ridge National Laboratory’s High Temperature Materials Laboratory (HTML).
The techniques can provide detailed characterizations of materials and allow components to be tested for heat and stress effects more effectively as part of the industry’s preparation for new emissions mandates that come into effect in the US in 2007. Under the new laws, a 90% reduction of nitrogen oxide, NOx, and particulates from diesel vehicles will be required.
“Environmental Protection Agency regulations are pushing emissions control technology very hard,” explains Arvid Pasto, director of the HTML, “so that engine and emissions control equipment manufacturers require access to very sophisticated tools to develop this technology. Fortunately, our user facilities are well equipped to help them.”
Diesel engine-maker Cummins, for instance, has used HTML’s analytical capabilities to better understand the properties of materials used in exhaust after-treatment systems. In addition to studying how catalysts can be adversely affected by sulfur and other gaseous exhaust components, Cummins and HTML have worked together to characterize the fatigue life of cordierite diesel soot filters, which remove more than 98% of particulate emissions from diesel exhaust. These exhaust after-treatment devices are critical to meeting upcoming emissions requirements.
Another company Industrial Ceramic Solutions, of Knoxville, Tennessee, used HTML’s scanning electron microscope facility to analyse material being developed for ceramic-fibre diesel particulate exhaust filters. The original material did not function as well as competing products and had a tendency to crack. The tests revealed that the fabrication process was to blame and ICS has modified its process to improve the product.
‘The sophisticated electron microscopy at HTML allowed our small business to literally look inside of the ceramic fiber filter media at thousands of times magnification,’ said Richard Nixdorf, ICS president and CEO. ‘This information led ICS to solutions that eliminated micro-cracking and moved our filter-media strength far beyond what the diesel exhaust filter application demanded.
 Regular readers will recall my mention of the Kuzyk Quantum Gap a few days ago and how Intute Spotlight would be covering news on how Kuzyk himself is closing the gap.
Regular readers will recall my mention of the Kuzyk Quantum Gap a few days ago and how Intute Spotlight would be covering news on how Kuzyk himself is closing the gap.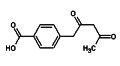 Spam comes in all shapes and forms, so I am always suspicious when two emails identical in content and with attachments arrive that purport to be from two different correspondents. However, two such messages arrived this morning one claiming to come from a Dr Suhasini Bhatnagar, the other from Aarif Khatri. Normally, I’d let my spam filter do its job and trash such messages, but my interest was piqued by the subject line, which read “help regarding synthesis”. Often spam arrives with two random words stuck together that are supposed to beat spam filters, but three is rare and even less frequent are subject lines that make logical sense and simultaneously are pertinent to my interests.
Spam comes in all shapes and forms, so I am always suspicious when two emails identical in content and with attachments arrive that purport to be from two different correspondents. However, two such messages arrived this morning one claiming to come from a Dr Suhasini Bhatnagar, the other from Aarif Khatri. Normally, I’d let my spam filter do its job and trash such messages, but my interest was piqued by the subject line, which read “help regarding synthesis”. Often spam arrives with two random words stuck together that are supposed to beat spam filters, but three is rare and even less frequent are subject lines that make logical sense and simultaneously are pertinent to my interests. A new type of radio frequency identification (RFID) sensor for gaseous molecules has been created based on a standard RFID tag coated with a chemically sensitive film at low cost. The use of multivariate analysis allows these new RFID sensors to be used to identify and quantify vapours important to industrial, in health, law enforcement, and of security applications.
A new type of radio frequency identification (RFID) sensor for gaseous molecules has been created based on a standard RFID tag coated with a chemically sensitive film at low cost. The use of multivariate analysis allows these new RFID sensors to be used to identify and quantify vapours important to industrial, in health, law enforcement, and of security applications.