 Almost £2 billion (about $3.7 billion) is needed to refurb school chemistry laboratories and help ensure British science remains viable, according to the Royal Society of Chemistry. If the money is not ring-fenced vital plans to upgrade the labs will fall a quarter of a century behind government targets.
Almost £2 billion (about $3.7 billion) is needed to refurb school chemistry laboratories and help ensure British science remains viable, according to the Royal Society of Chemistry. If the money is not ring-fenced vital plans to upgrade the labs will fall a quarter of a century behind government targets.
The RSC’s chief executive Richard Pike said today: ‘Our case for faster action to improve school labs, and to assign money to the task, is powerful and incontrovertible. Without something being done to address this slippage Britain could drift to the margins of world science as potential young talent goes unexploited.’ If the government fails to deliver, then this will harm the UK’s competitiveness, the RSC claims in a report on the state of Britain’s school labs, published today:
Pike adds that, ‘There is an acute national need to promote chemistry attractively and inspirationally at school. We have a duty to stimulate the science interest of young people and it is glaringly obvious that sub-standard, dull and bedraggled laboratories will sour the appeal of chemistry and deter them from engaging in the subject successfully.”
With many university chemistry departments hanging by a shoestring and most recently the physics department at Reading University facing closure, it seems timely to remind the government of the importance of science education. Without the much-need upgrades to school labs, our children will not gain the necessary skills so effectively, will not develop a love of science, and will opt for alternative careers. Ultimately, the UK’s science base and so its international competitiveness on many fronts will suffer.
Of course, with the government also intending to abandon assessed coursework for science, maybe we won’t need those labs at all.
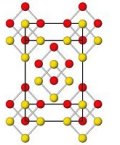 Nature described this finding as “surprising, elegant, and entirely useless”. Well, the journal is half right. Solid elemental oxygen is not thought to exist anywhere on earth or even elsewhere in the universe under the immense pressures created by Malcolm McMahon and Paul Loubeyre. They and their colleagues put the squeeze on solid oxygen, which forms deep red crystals at above a million atmospheres. They used various techniques to determine the structure of this new material and found that oxygen atoms team up to form clusters of eight in the solid. A seemingly esoteric discovery you might think.
Nature described this finding as “surprising, elegant, and entirely useless”. Well, the journal is half right. Solid elemental oxygen is not thought to exist anywhere on earth or even elsewhere in the universe under the immense pressures created by Malcolm McMahon and Paul Loubeyre. They and their colleagues put the squeeze on solid oxygen, which forms deep red crystals at above a million atmospheres. They used various techniques to determine the structure of this new material and found that oxygen atoms team up to form clusters of eight in the solid. A seemingly esoteric discovery you might think. Is your browser so locked down that you can’t install any plugins or enable Java? Firewall refusing to cooperate with your molecules? Antivirus screaming at your structural efforts?
Is your browser so locked down that you can’t install any plugins or enable Java? Firewall refusing to cooperate with your molecules? Antivirus screaming at your structural efforts?