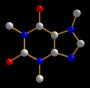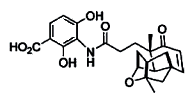
The authors of a paper published in this week’s Nature claim to have srtuck a blow against the rising tide of antibiotic resistance. Jun Wang and colleagues at Merck’s Research Laboratories, in Rahway, New Jersey, have found a potent antibiotic from a microbial fungus, which they demonstrate kills many Gram-positive bacteria, including the current media darlings methicillin-[or multiple]-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).
The new antibiotic, platensimycin, was just one of 250,000 natural product extracts they screened from a strain of the South African soil microbe Streptomyces platensis.
What makes platensimycin so interesting is its mode of action, which is completely different from any other antibiotic. This compound kills bacteria by blocking the enzymes involved in the synthesis of fatty acids, the building blocks of lipids. No existing antibiotics target fatty-acid synthesis in this way. The antibiotic clears Staph infection without any apparent side effects and it or an analog is now very likely to be pursued by the pharmaceutical industry.
Only two new classes of antibiotics have been found since the mid-1960s, the linezolid type (oxazolidinones) and daptomycin (lipopeptide), most of the others we use were found in the 1940s and 1950s and target specific bacterial biochemical processes, such as cell wall construction, their DNA and proteins. This limited arsenal allowed bacteria to evolve resistance to pretty much every antibiotic, which is why a new class is so keenly sought.
According to Eric Brown of McMaster University, in Hamilton, Ontario, “The report reads like a textbook of modern antibacterial drug discovery, beginning with a screen of 250,000 extracts from drug-producing microorganisms. What follows is a series of elegant studies, spanning bacterial genetics, biochemistry, pharmacology and structural biology, and leading to the discovery of a small molecule.”
Ever the cynic I cannot help worry that regardless of how novel this compound is at this point in its clinical history, as with all of its predecessors, bacteria are likely to evolve just as effective defences as their cousins once we begin to use platensimycin in medicine. That was the lesson we should have learned the very first time the prototypical antibiotic penicillin was used! Today’s wonder drug quickly becomes tomorrows acronym, give it a few years and the headlines will be screaming about PRSA, you can bet on it.
Nature, 2006, 441, 358