During the last decade or so (coincident with the development of open access journal PLoS One, as it happens), the paradigm of “open”, as in open innovation, has changed the way R&D is organised and run in countless high-technology firms. However, the open innovation model has to be adapted and modified to fit specific areas. French researchers have now surveyed managers across the UK’s biopharmaceutical sector at the small-medium enterprise (SME) level to identify what needs to be improved to open innovation still further.
Calin Gurau and Frank Lasch of the GSCM-Montpellier Business School, explain that closed innovation is the classical business model, the one in which a company carries out its R&D and market research entirely in secret, allowing no other eyes to see the products heading for patent and locking down any sub-contractors with tyrannical non-disclosure agreements. Open innovation, on the hand, has emerged as markets and technology have changed. The team points out that the biopharmaceutical sector has developed significantly in the last 30 years.
Until the 1970s, innovation was the realm of big pharma, but access by SMEs and academic spin out companies to relatively inexpensive instrumentation and robotic equipment has meant that almost anyone with a small army of PhDs can innovate to some degree. Similarly, as academics turned to wealth creation so many of the brightest individuals in the corporate world jumped ship to create their own start-ups with a similar thought in mind. It was not quite kitchen bioscience, but the post-genomic era gave another boost to SMEs hoping to get on the pharma ladder and nip at the ankles of the multinationals.
Of course, it quickly became apparent, as it did in the dot.com era, that SMEs cannot necessarily cover all aspects of R&D to compete with big pharma. Thus emerged the open innovation concept. The bright young things whether emerging fresh from academia or shrugging off the corporate cocoon began to realise that their skills could be better served and make them more money by engaging in collaborations with big pharma to provide complementary resources to finalise the R&D operations and bring high-value products to market.
Gurau and Lasch suggest that there are four reasons why open innovation was not only inevitable but essential to the ongoing success of the industry as a whole:
- The complexity of the innovation, necessitating resources that cannot be developed internally by a single organisation
- The dynamics of competition forces the organisation to accelerate the innovation process
- The risk of innovation, which requires a combination of expertise and flexibility that can be often realised only through the collaboration of various highly specialised firms in an innovation network
- The specific innovative conditions that cannot be reproduced in large organisations
The (un)conventional perspective on open innovation in pharma is that it works in a similar way to the open source software movement. This model is already in place. Researchers participate and have the right to use freely the discoveries developed within the group, but also being obliged to make freely available to the group any improvement they make to the initial innovation. The Australian non-profit organisation Cambia is a good example of an open innovator as is Open Notebook Science (albeit with a greater academic focus). The website Innocentive.com, initially an initiative of the pharmaceutical company Eli Lilly about which I wrote in my Catalyst column on ChemWeb.com back in the early 2000s, represents an example of an open market for innovative solutions.
A third approach to open innovation sees adaptation of the value co-creation concept in which value is exchanged as a partnership between two parties evolves. The exchange process can sometimes determine the gradual development of a relationship that transforms the open exchange process into a stable business relationship, the researchers suggest.
“The issue of open innovation is particularly significant for the SMEs from high-technology sectors. In the biopharmaceutical sector, the length and the complexity of the R&D process, coupled with the low level of resources of small biotech firms, forces these organisations to search outside sources of innovative expertise and technology,” the researchers conclude.
 Calin Gurau, & Frank Lasch (2011). Open innovation strategies in the UK biopharmaceutical sector Int. J. Entrepreneurial Venturing, 3 (4), 420-434
Calin Gurau, & Frank Lasch (2011). Open innovation strategies in the UK biopharmaceutical sector Int. J. Entrepreneurial Venturing, 3 (4), 420-434
 The Q4 issue of the Cutting Edge of Chemistry (PDF) newsletter which I produce for ThomsonReuters is now online. In this latest issue:
The Q4 issue of the Cutting Edge of Chemistry (PDF) newsletter which I produce for ThomsonReuters is now online. In this latest issue: The role of the drug discovery chemist has changed significantly over the past 50 years – workflows have been reinvented while the same goals remain to find and test novel molecules that can reach and act on disease targets.
The role of the drug discovery chemist has changed significantly over the past 50 years – workflows have been reinvented while the same goals remain to find and test novel molecules that can reach and act on disease targets.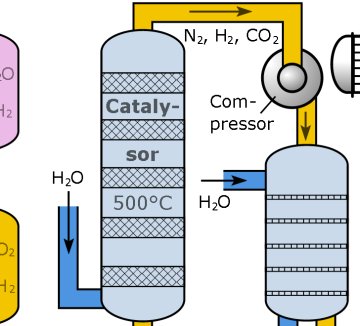 It is a perennial on almost every chemistry course: the Haber-Bosch process for making ammonia. A potassium-doped iron catalyst is heated to several hundred degrees and high pressure nitrogen and hydrogen gas mixed over it to generate ammonia, which is then fed into fertiliser production. The H-B process very effectively traps and fixes nitrogen from the air, and is the industrial equivalent of nitrogen fixation by legumes and living other plants. The process, first demonstrated in 1909, has fed millions of people through the agricultural revolution of the twentieth century. You might say it has allowed our species to become so successful that it now numbers 7 billion…
It is a perennial on almost every chemistry course: the Haber-Bosch process for making ammonia. A potassium-doped iron catalyst is heated to several hundred degrees and high pressure nitrogen and hydrogen gas mixed over it to generate ammonia, which is then fed into fertiliser production. The H-B process very effectively traps and fixes nitrogen from the air, and is the industrial equivalent of nitrogen fixation by legumes and living other plants. The process, first demonstrated in 1909, has fed millions of people through the agricultural revolution of the twentieth century. You might say it has allowed our species to become so successful that it now numbers 7 billion…