 Solid state NMR is unlocking the secrets of compounds found in natural membranes from frogs’ legs to human lungs that could lead to an entirely new class of antibiotic drugs. The compounds in question are antimicrobial peptides (AMPs) and they have been detected in every living creature studied so far. AMPs act as a first line chemical defence system in a huge range of organisms and could provide a novel approach to defeating drugs resistance in bacteria.
Solid state NMR is unlocking the secrets of compounds found in natural membranes from frogs’ legs to human lungs that could lead to an entirely new class of antibiotic drugs. The compounds in question are antimicrobial peptides (AMPs) and they have been detected in every living creature studied so far. AMPs act as a first line chemical defence system in a huge range of organisms and could provide a novel approach to defeating drugs resistance in bacteria.
“Our overall mission is to use the kind of basic physical data we obtain from NMR to help interpret biological functions,” team leader Ayyalusamy Ramamoorthy of the University of Michigan explains. As with most discoveries of this nature, it will be several years before any clinical trials for specific health problems or diseases are complete. “How it works against viruses are under investigation in other labs,” Ramamoorthy told me.
You can find out more about AMPs as the front line defenders in the latest issue of SpectroscopyNOW.
 The faint glow from a single molecule combined with a stretch from “magnetic tweezers” could help scientists get a grip on how viruses that infect bacteria, so-called bacteriophages pack up their DNA. The research could lead to a resurgence of interest in the West for a potent treatment for infection that uses
The faint glow from a single molecule combined with a stretch from “magnetic tweezers” could help scientists get a grip on how viruses that infect bacteria, so-called bacteriophages pack up their DNA. The research could lead to a resurgence of interest in the West for a potent treatment for infection that uses  Face recognition is the most obvious approach to identification but it suffers from a major drawback – shadows and bad lighting. If there is inconsistent lighting in a room or on a face then it becomes difficult to produce reproducible digital image of the face for face recognition algorithms to work with. Now, researchers in China have turned to near infra-red to help computers cope with variable lighting conditions and so recognize even the most shadowy of faces.
Face recognition is the most obvious approach to identification but it suffers from a major drawback – shadows and bad lighting. If there is inconsistent lighting in a room or on a face then it becomes difficult to produce reproducible digital image of the face for face recognition algorithms to work with. Now, researchers in China have turned to near infra-red to help computers cope with variable lighting conditions and so recognize even the most shadowy of faces. Many of the health claims of herbal medicine bear fruit for the pharmaceutical industry, leading to new drugs that are more potent and more targeted than the original remedy. In Traditional Chinese medicine there are many health claims for the likes of Ginkgo biloba and many other remedies that might bear closer scrutiny. Now, pharmaceutical chemist David Barlow and colleagues Peter Hylands and Thomas Ehrman at King’s College London have undertaken the biggest study yet of the active ingredients in TCM and used an analytical system known as a multiple decision tree technique, called Random Forest, to unearth the root of the activity of the natural products in TCM.
Many of the health claims of herbal medicine bear fruit for the pharmaceutical industry, leading to new drugs that are more potent and more targeted than the original remedy. In Traditional Chinese medicine there are many health claims for the likes of Ginkgo biloba and many other remedies that might bear closer scrutiny. Now, pharmaceutical chemist David Barlow and colleagues Peter Hylands and Thomas Ehrman at King’s College London have undertaken the biggest study yet of the active ingredients in TCM and used an analytical system known as a multiple decision tree technique, called Random Forest, to unearth the root of the activity of the natural products in TCM.
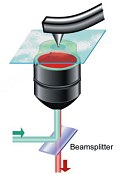 Swiss chemists have devised a new approach to the familiar analytical technique of Raman spectroscopy that allows them to investigate the structure of individual molecules.
Swiss chemists have devised a new approach to the familiar analytical technique of Raman spectroscopy that allows them to investigate the structure of individual molecules. Advocates of nuclear power point to recent advances in waste storage materials that could allow the radioactive byproducts of the nuclear industry to be stored safely and indefinitely in ceramics rather than glass. Whereas those not in favour of splitting atoms to produce almost limitless energy point out that even vitrified nuclear waste will represent an ongoing problem for thousands of years.
Advocates of nuclear power point to recent advances in waste storage materials that could allow the radioactive byproducts of the nuclear industry to be stored safely and indefinitely in ceramics rather than glass. Whereas those not in favour of splitting atoms to produce almost limitless energy point out that even vitrified nuclear waste will represent an ongoing problem for thousands of years. Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.
Two back-to-back papers in the well-known chemistry journal Angewandte Chemie recently could have potentially serious consequences for the pharmaceutical industry, because they reveal what the authors claim are inherent ambiguities in the crystalline forms of aspirin.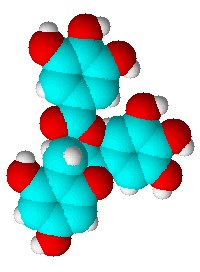 Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria.
Researchers from Slovenia have used spectroscopy to home in on the active site of an essential bacterial enzyme, DNA gyrase. They say they now understand more clearly how a compound found in green tea, EGCG, which is a health-boosting antioxidant, works to kill bacteria.