 Viagra could be the cure mile-high clubbers have been waiting for. Apparently, not only can the drug help men keep up appearances at any altitude, new research could lead to its extension to other areas of medicine, such as the treatment of jet lag.
Viagra could be the cure mile-high clubbers have been waiting for. Apparently, not only can the drug help men keep up appearances at any altitude, new research could lead to its extension to other areas of medicine, such as the treatment of jet lag.
Apparently, researchers in Brazil simulated jet lag in hamsters by exposing them to light out of phase with their natural body clock, giving the little beasts the feeling of having flown from Paris to New York on the red-eye day after day. The result was that the hamsters’ circadian cycle got so skewed that they would mount their wheels and run 14 hours before they should each night.
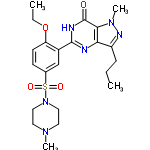
However, Diego Golombek and colleagues from the Quilmes National University in Buenos Aires, figured that a quick shot of Viagra (sildenafil citrate, sildenafil without its counter ion is shown) might work wonders for the hardy little creatures. In fact a 70 mg dose of the ED drug, reduced the jet lag recover period for the animals to just over a week, compared with the two weeks it took the Viagra-free hamsters to recover. What a relief.
Circadian clocks regulate the timing of biological functions in almost all higher organisms, say Cornell University and Dartmouth College researchers writing independently in the journal Science, this week. Anyone who has flown through several time zones knows the jet lag that can result when this timing is disrupted, they say.
Now, the Cornell and Dartmouth scientists believe they can explain the biological mechanism behind how circadian clocks sense light through a process that transfers energy from light to chemical reactions in cells. Whether or not this research tells us if you should keep your eyes closed during or after entry into the mile-high club or how to disguise why you are not suffering jet lag after a long trip is a different matter.
InChI=1/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)/f/h23H
When nicotine binds to a neuron, how does the cell know to send the signal that announces a smoker’s high? A recently determined crystal structure of a key player in the process suggests that a sugar molecule has a simple mechanical role acting as a hinge to open a gate in the cell membrane. The research might one day lead to new treatments for drug addiction, depression, epilepsy, schizophrenia, and other disorders.