Diethylstilbestrol (DES) is a synthetic nonsteroidal estrogen that was first synthesized in 1938. Human exposure to DES has allegedly occurred through diverse sources, such as dietary ingestion from supplemented cattle feed and medical treatment for certain conditions, including breast and prostate cancers.
From 1940 to 1970, DES was actually given to pregnant women in the belief that it would reduce the risk of pregnancy complications and losses. In 1971, DES was shown to cause a rare vaginal tumour in girls and young women who had been exposed to this drug in utero and the US FDA subsequently withdrew DES from use in pregnant women.
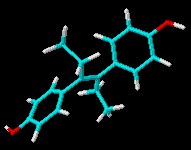 Dan Lednicer offered us a guest editorial some time ago that mentioned the ill-fated drug diethyl stilbestrol (DES) and the toxicity of this and other compounds. Here’s the 3D chemical structure of DES for recent visitors to Sciencebase who were looking for it!
Dan Lednicer offered us a guest editorial some time ago that mentioned the ill-fated drug diethyl stilbestrol (DES) and the toxicity of this and other compounds. Here’s the 3D chemical structure of DES for recent visitors to Sciencebase who were looking for it!
Diethylstilbestrol is an orally active non-steroidal estrogen first made in 1938 and originally approved for use in gonorrheal vaginitis, atrophic vaginitis, for menopausal symptoms, and in postpartum lactation suppression to prevent breast engorgement.
However, in 1971 it was found to be a teratogen – causing birth defects – when given to pregnant women and later a carcinogen. It is currently used only in veterinary practice at very low (hypocarcinogenic) doses for treating female canine incontinence.