TL:DR – David Bradley Science Writer provides the molecular structure of bleach
It seems to be something of an obsession with sciencebase.com visitors, but for some nothing would delight them more than to discover the systematic name for bleach, the chemical formula for bleach, the molecular structure of bleach, or to find a molecular model of bleach.
Well, the structure of bleach depends on what you mean by bleach. There are various kinds of chemical agents that will “remove” the colour from pigmented materials. Perhaps most commonly considered bleach is the liquid mixture that contain sodium hypochlorite (NaOCl, Na for sodium, O for oxygen, Cl for chlorine atoms). This is the active ingredient in the common household bleaches that “get right up under the rim” and “kill all known germs”. It is the “hypo” part of the name that gives it its bleaching properties without the O it would simply be sodium chloride or common salt, and if the O were in the wrong place – NaClO – we’d have sodium chlorite (a less powerful oxidising agent used to whiten textiles without harming cellulose fibres).
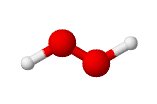 There are now several non-chlorine bleaches available for domestic use such as those containing hydrogen peroxide (H2O2), this material is well known as a bleach and often associated with people who allegedly have more fun). To the right you can see a simple molecular model of hydrogen peroxide.
There are now several non-chlorine bleaches available for domestic use such as those containing hydrogen peroxide (H2O2), this material is well known as a bleach and often associated with people who allegedly have more fun). To the right you can see a simple molecular model of hydrogen peroxide.