 On Christmas Day 2006, I posted a blog about how snowflakes are not all different and some of the science underlying the formation of snowflakes. The American Chemical Society had a nice infographic at the time showing the principles of snowflake formation (PDF here). There’s no snow around here, but this is Britain, the weather could change at any moment and although we don’t quite have the four seasons in one day they get in New Zealand, give it a day or two and a warm spell can become a cold snap almost overnight.
On Christmas Day 2006, I posted a blog about how snowflakes are not all different and some of the science underlying the formation of snowflakes. The American Chemical Society had a nice infographic at the time showing the principles of snowflake formation (PDF here). There’s no snow around here, but this is Britain, the weather could change at any moment and although we don’t quite have the four seasons in one day they get in New Zealand, give it a day or two and a warm spell can become a cold snap almost overnight.
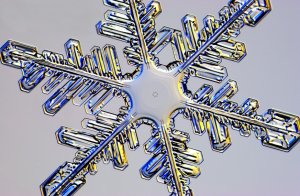
Snowflakes have at their heart a minute grain of dust that was once floating in a cloud, this speck of dust is the nucleation centre around which water vapour from the atmosphere can condense and if it is cold enough crystallise as ice. As with any crystallisation process it follows a symmetry intrinsic to the atoms or molecules from which the crystal is formed. In the case of water, the underlying symmetry is hexagonal symmetry. There’s more on this in the snow crystal primer.
Caltech’s Kenneth Libbrecht’s is the snowflake guru, here’s a video montage of his snow crystal gallery set to the tune of A Guy Called Gerald by Humanity (Borngraber & Struver Remix):
One question that puzzled me as a child is how each of the six arms “knows” to grow in the same way? Well, they’re growing under almost identical conditions so that might be expected, except, of course, that pretty drawings and diagrams aside, the six arms of snow crystals don’t actually grow symmetrically at all. At first glance they might look nice and symmetrical but under the microscope a single snow crystal will be seen to be far less than perfect in its symmetry. Symmetrical snow crystals are very rare except on Christmas cards. Speaking of which I abandoned my Christmas rant about octagonal and pentagonal snowflakes which are common in those pretty pictures but are physically impossible in the real world of snow because of symmetry constraints.