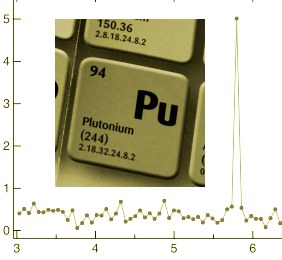
 After a half century of trying, spectroscopists have finally pinned down the nuclear magnetic resonance (NMR) spectrum of plutonium-239. The finding will have implications for future studies of the solid-state physics of this important nuclear fuel and might point the way to improved approaches to the long-term storage of nuclear waste as well as the development of nuclear-powered spaceflight.
After a half century of trying, spectroscopists have finally pinned down the nuclear magnetic resonance (NMR) spectrum of plutonium-239. The finding will have implications for future studies of the solid-state physics of this important nuclear fuel and might point the way to improved approaches to the long-term storage of nuclear waste as well as the development of nuclear-powered spaceflight.
You can read the full story in my Chemistry World news update today. However, I have some additional comments on the technicalities of the work from team leader Georgios Koutroulakis of Los Alamos National Laboratory.
Koutroulakis points out that working with highly pure plutonium(IV) oxide allowed them to achieve what others have been unable to do in fifty years. First, the non-magnetic ground state of the particular system studied helped them overcome the problem of the exceedingly short relaxation time (and huge resonance shift) of the plutonium-239 nucleus. “The energy gap between the ground state and the first excited magnetic state is quite large for the spins to dissipate their energy to the “lattice” in order to relax and, so, the relaxation time becomes reasonable and we can detect the signal,” he told me.
Cooling the system to close (4 Kelvin) to liquid helium temperatures was critical too. “At higher temperature it’s much easier for the spins to “hop” to higher energy states and consequently the relaxation becomes faster,” he explains. “For this reason exactly we went to low temperature, to assure that we were on average on the magnetic ground state hoping that T1 will be long enough and the shift small enough for us to be able able to observe the NMR signal, as proved to be the case.”
As to what they did differently from the many groups that have tried to obtain plutonium NMR spectra in the past: “The main limitation has been the very short T1/huge shift and, of course, the fact that people didn’t know where to look in terms of field-frequency without knowing the value of gamma-n. So, most of the relevant efforts have been concetrated on finding Pu-based materials in which, for one reason or another, these limitations are side-stepped, for instance systems with superconducting or antiferromagnetic ground states, but nothing had worked so far. We thought, contrary maybe to most people in the field, that working on a very pure sample of plutonium(IV) oxide in which Pu ions show a non-magnetic ground state we might have a chance to overcome the aforementioned limitations and observe the signal.”
Koutroulakis adds that his team was “clever” to choose the right system in which to look for the Pu NMR signal. “This, in combination with our very well-equipped NMR laboratory and exceptional knowhow (most NMR chemists, for example, cannot sweep their magnetic field and most solid-state NMR physicists don’t have access to such high-quality Pu-based samples), a lot of perseverance and patience (we had to try very long, time-consuming field sweeps with lots of different experimental/detection parameters), and a touch of luck of course (actually, we detected the signal when we were about to give up!) led to our finding.”
I asked NMR expert Ian Farnan of the University of Cambridge to comment on the importance of the work, he told me the first mention of the results was at a conference back in February. “It is an important breakthrough in actinide science as it provides an element specific insight into the local structural and magnetic environment of Pu in a range of materials,” Farnan says. “We have thought of searching for its resonance ourselves but have been put off by the wide frequency range and the low likelihood of lack of success. The Los Alamos group has to be congratulated and thanked because it is a great effort that opens up the field,” he adds.
Farnan and his colleagues have concentrated on the observation of NMR active ligands, such as oxygen-17 attached to actinides such as Pu using the magic-angle spinning (MAS) technique. This technique provides the high resolution that is required to distinguish subtle structural differences between local atomic environments in solids. “We are adapting the NMR equipment at the European Commission Joint Research Centre Institute for Transuranic Elements for 239Pu observation,” he adds. “This possibility will be available to scientists through the EC FP7 programme EURACT-NMR.”
![]() H. Yasuoka, G. Koutroulakis, H. Chudo, S. Richmond, D. K. Veirs, A. I. Smith, E. D. Bauer, J. D. Thompson, G. D. Jarvinen, D. L. Clark (2012). Observation of 239 Pu Nuclear Magnetic Resonance Science, 336, 901-904 : 10.1126/science.1220801
H. Yasuoka, G. Koutroulakis, H. Chudo, S. Richmond, D. K. Veirs, A. I. Smith, E. D. Bauer, J. D. Thompson, G. D. Jarvinen, D. L. Clark (2012). Observation of 239 Pu Nuclear Magnetic Resonance Science, 336, 901-904 : 10.1126/science.1220801