On 21st May 2005, medicine celebrated the bicentenary of the crystallization of morphine
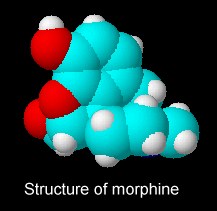 Since 1805, morphine and its derivatives have become the most widely used treatment for severe pain. Now more than 230 tons of morphine is used each year for medical purposes including pain relief for patients with chronic pain or advanced medical illness and post-operative analgesia. By the way, if you’re looking for a chemical structure for opium, you’re out of luck. Opium is not a single compound but an extract of the immature seeds of the opium poppy, Papaver somniferum. This extract contains up to 10% morphine, the opiate alkaloid, which is processed to produce diamorphine, better known as heroin. The resin also includes codeine and non-narcotic alkaloids, such as papaverine and noscapine.
Since 1805, morphine and its derivatives have become the most widely used treatment for severe pain. Now more than 230 tons of morphine is used each year for medical purposes including pain relief for patients with chronic pain or advanced medical illness and post-operative analgesia. By the way, if you’re looking for a chemical structure for opium, you’re out of luck. Opium is not a single compound but an extract of the immature seeds of the opium poppy, Papaver somniferum. This extract contains up to 10% morphine, the opiate alkaloid, which is processed to produce diamorphine, better known as heroin. The resin also includes codeine and non-narcotic alkaloids, such as papaverine and noscapine.
Despite 200 years of increasingly frequent use, however, even the medical uses of morphine still present problems, such as severe nausea, itching, and constipation.
Moss has been invited to speak at the Einbeck morphine-commemorative conference in May on the relationship between morphine and a drug known as methylnaltrexone — a peripheral opiate antagonist developed at the University of Chicago — which can prevent many of these troubling side effects.
Moss’s lecture, “Morphine’s secrets revealed,” will focus on how methylnaltrexone enables scientists to distinguish between the central analgesic effects of morphine and its peripheral side effects. Check out the Chemspider page for morphine.
Discovery of morphine
Morphine was discovered by Freidrich Wilhelm Adam Serturner (1783-1841), an obscure, uneducated, 21-year-old pharmacist’s assistant with little equipment but loads of curiosity.
Serturner wondered about the medicinal properties of opium, which was widely used by 18th-century physicians. In a series of experiments, performed in his spare time and published in 1806, he managed to isolate an organic alkaloid compound from the resinous gum secreted by Papaver somniferum — the opium poppy.
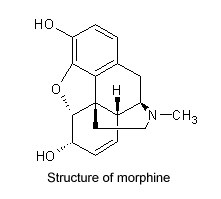 Serturner found that opium with the alkaloid removed had no effect on animals, but the alkaloid itself had ten times the power of processed opium. He named that substance morphine, after Morpheus, the Greek god of dreams, for its tendency to cause sleep. He spent several years experimenting with morphine, often on himself, learning its therapeutic effects as well as its considerable dangers. Although his work was initially ignored, he recognized its significance. “I flatter myself,” he wrote in 1816, that “my observations have explained to a considerable extent the constitution of opium, and that I have enriched chemistry with a new acid (meconic) and with a new alkaline base (morphium), a remarkable substance.”
Serturner found that opium with the alkaloid removed had no effect on animals, but the alkaloid itself had ten times the power of processed opium. He named that substance morphine, after Morpheus, the Greek god of dreams, for its tendency to cause sleep. He spent several years experimenting with morphine, often on himself, learning its therapeutic effects as well as its considerable dangers. Although his work was initially ignored, he recognized its significance. “I flatter myself,” he wrote in 1816, that “my observations have explained to a considerable extent the constitution of opium, and that I have enriched chemistry with a new acid (meconic) and with a new alkaline base (morphium), a remarkable substance.”
As he predicted, chemists and physicians soon grew interested in his discoveries. Serturner’s crystallization of morphine was the first isolation of a natural plant alkaloid. It sparked the study of alkaloid chemistry and hastened the emergence of the modern pharmaceutical industry.
Other researchers soon began to isolate similar alkaloids from organic substances, such as strychnine in 1817, caffeine in 1820 and nicotine in 1828. In 1831, Serturner won a lucrative prize for the discovery.
In 1818, French physician Francois Magendie published a paper that described how morphine brought pain relief and much-needed sleep to an ailing young girl. This stimulated widespread medical interest. By the mid-1820s morphine was widely available in Western Europe in standardized doses from several sources, including the Darmstadt chemical company started by Heinrich Emanuel Merck.
By the 1850s the first reliable syringes were developed and injected morphine became a standard method of reducing pain during and after surgery. Since then, various delivery systems for morphine have been developed, including epidural injection and pumps that allow patient-controlled analgesia.
Although morphine was originally touted as a cure for many maladies, even for opium addiction, by the 1870s physicians had become increasingly aware of its own addictive properties. Ironically, C.R. Alder Wright, a chemist at a London hospital who was searching for a non-addictive alternative to morphine, came up with a more potent narcotic, diacetylmorphine, in 1874.
Heinrich Dreser, a chemist at Bayer Laboratories developed and tested Wright’s new semi-synthetic drug on animals, humans, and most notably himself. Finding that it was a powerful painkiller and appeared effective for a variety of respiratory ailments, Bayer began producing and marketing this drug as an analgesic and a “sedative for coughs” in 1898. Because of its “heroic” ability to relieve pain, they called it heroin.
The medical profession initially welcomed the new drug but soon recognized it’s addictive potential. In 1913, Bayer halted production, edited the drug out of their official company history and focused instead on marketing their second blockbuster drug, aspirin.
Discovery of Methylnaltrexone
Yearly, more than 500,000 patients with advanced cancer depend on powerful opioid-based pain relievers such as morphine, or its derivatives OxyContin or Percocet, for pain relief. One side effect of all narcotic pain relievers is severe constipation, which can be so distressing that many patients discontinue their pain medication.
To solve this problem, the late Leon Goldberg, a University of Chicago pharmacologist, developed methylnaltrexone (MNTX). In order to help a friend with morphine-induced constipation, Goldberg modified naltrexone, an established drug that blocks the effects of morphine, so that it could no longer cross the protective barrier that surrounds the brain. Consequently, it did not interfere with morphine’s effect on pain, which is centred in the brain, but it did block morphine’s effects on gut motility, which are mediated by receptors in the gastrointestinal tract.
Goldberg’s university colleagues continued to develop the compound, testing it in animals and performing the initial human safety trials and clinical studies in volunteers and patients.
The University of Chicago licensed the MNTX technology to UR Labs, Inc. and in 2001, Progenics Pharmaceuticals of Tarrytown, NY, sub-licensed the worldwide exclusive rights to develop MNTX from UR Labs. One phase 3 trial of MNTX for treatment of opioid-induced constipation in patients with advanced medical illness has been completed and results from a second trial were reported May 17 at the American Society of Clinical Oncology annual meeting. Progenix has a target date of New Drug Application submission in late 2005.
Meanwhile, Moss and his University colleagues have identified multiple uses of MNTX, beyond the original discovery by Goldberg. Some of these additional uses of MNTX include treatment of post-operative bowel dysfunction (a serious impairment of the gastrointestinal tract following surgery), opioid-induced itching, urinary retention, and possibly HIV.
Opiates appear to increase the ability of HIV to infect certain immune system cells. In 2003, Moss reported that very small amounts of methylnaltrexone blocked these increases. “If our studies are borne out in future clinical trials, methylnaltrexone may improve the care of patients who take opioids for pain caused by AIDS,” he said.
“Two hundred years after Serturner’s work, we continue to learn a great deal about morphine,” Moss said. “The ability to facilitate pain relief while minimizing side effects is both conceptually important and very relevant to patient care.”