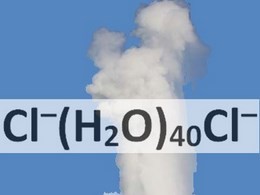
An international team of chemists has looked at the seemingly esoteric subject of microsolvation of chloride pairs. They have found that in computer models, at least, it takes about forty water molecules to make a pair of negative chloride ions stay together.
I asked team leader Utah University’s Alexander Boldyrev about the wider importance of this study which could affect geochemical research, atmospheric and climate science as well as having implications for chemical engineering.
Steam, containing dissolved alkali halide ions, can form clusters of Na+(H2O)n and Cl-(H2O)n. Based on our results it is now possible to investigate hydrophobic interactions, i.e., interactions between the ions surrounded by water molecules that could clarify the physical picture of condensation in real steam. In our work we traced the formation of chloride-chloride pair in the gas phase. We think this yields an adequate picture of physical processes that occur during steam condensation in the presence of dissolved ions.
Industrial steam and geysers
We also believe that the results of our calculations indicate that the chloride-chloride pair plays the role of a trap for water molecules in steam. Probably, the increased content of dissolved chloride in the first condensate of industrial steam serves as experimental support for the appearance of stable anion pairs and their active role in nucleation observed in steam. Thus, the presence of solvated ions and pairs of ions in the steam will accelerate condensation of the industrial steam and that should be taken by engineers into account for steam system improvements. Also, we think that such stable gaseous clusters as Cl-(H2O)40Cl- can be present in geyser’s steam (boiling of the pressurized water, containing chlorides, results in the geyser effect of hot water and steam spraying out of the geyser’s surface vent – a hydrothermal explosion) and accelerate geyser’s steam condensation.
Atmospheric water vapour
Sea water becomes airborne in the form of droplets formed at the sea surface by the action of waves. These droplets can be carried by the wind, and they interact with other constituents of the atmosphere. On the surface of the small water droplets, halide anions can be converted into halogen atoms by absorption of light and the air—water interface serves to increase certain reaction probabilities. One example is the oxidation of Cl− and Br− by OH radicals or O3 that can occur at the air—water interface, with mechanisms different from those in the bulk phase, leading to natural ozone depletion in the stratosphere. The discovered in our work stable [Cl2(H2O)36]2− and [Cl2(H2O)40]2− clusters are greater than 1 nanometre, so they represent water nanodroplets doped with chlorides and can be thoroughly studied with the use of quantum chemistry. Thus it might be helpful in understanding the fundamental properties of aerosols, which remain among the largest uncertainties in climate science.