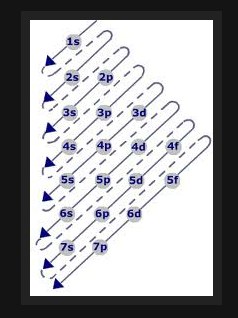
 Back in the 20th century, around the time quantum physics was emerging, Niels Bohr tried to explain the pattern of elements that make up the Periodic Table based on the order in which each atom’s “shells” fill with electrons. Unfortunately, the theoretical filling order governed by the so-called Aufbau principle doesn’t actually work experimentally and chemists had to adopt a sloppy version of the principle to make the Periodic Table fit the real-life chemistry rather than the theory.
Back in the 20th century, around the time quantum physics was emerging, Niels Bohr tried to explain the pattern of elements that make up the Periodic Table based on the order in which each atom’s “shells” fill with electrons. Unfortunately, the theoretical filling order governed by the so-called Aufbau principle doesn’t actually work experimentally and chemists had to adopt a sloppy version of the principle to make the Periodic Table fit the real-life chemistry rather than the theory.
This sloppy version of Bohr’s approach litters the chemical literature and textbooks across the globe. This one little lie leads to others as chemistry educators have to invent more and more elaborate kludges to explain why the electron filling does not in reality follow the order predicted by the principle.
Now, chemical philosopher Eric Scerri has exposed the sloppiness and suggests it is time for chemists to abandon the physicist’s 20th century explanation as to how their periodic table works and devise their own principle that fits not only the experimental data but is a worthy theory to make future predictions about real elements.
After all, wasn’t it Feynman who said: “It doesn’t matter how beautiful your theory is, it doesn’t matter how smart you are. If it doesn’t agree with experiment, it’s wrong.”